Abstract
Background
There is now great interest in using digital health tools to monitor patients' health status in real-world practice. Such tools often include electronic-patient-reported outcome (ePRO) systems in which symptoms questions are included into online interfaces for patient self-reporting, with real-time alerts triggered to the treating physician if severe symptoms or problems are reported. However, there is little information about the clinical utility and user perceptions of these systems, and this is particularly true in the area of hematology.
Objectives
This study investigates physicians' perceptions of usability and clinical utility of using remote ePROs in routine practice of patients with hematologic malignancies and explored implications in the delivery of patient care.
Patients and Methods
Remote ePROs are being gathered since December 2020 by the ALLIANCE Digital Health Platform, whose details of the development process have been previously described (Efficace F. et al., JMIR Res Protoc. 2021 Jun 1;10:e25271). Adult patients diagnosed with any hematologic malignancy are eligible to enter the platform, after having provided written informed consent. Aspects related to health-related quality of life (HRQoL), symptoms and medication adherence are assessed via validated PRO measures. The platform allows for real-time graphical presentation to physicians of individual patient symptoms and HRQoL outcomes. Based on a pre-defined algorithm, which includes the presence of clinically important problems and symptoms, the platform triggers automated alerts to the treating haematologists and medical staff. The definition of clinically important problems and symptoms is based on previously defined evidence-based thresholds (Giesinger J. et al., J Clin Epidemiol. 2020 Feb;118:1-8). We asked treating haematologists a feedback about their experience in using the platform, by an ad hoc web-survey consisting of 27 items covering several domains, including: usability and benefits, current use, evaluation of patient health-status, symptoms and adverse events, as well as physician-patient communication. We summarized characteristics of enrolled patients and treating haematologists by proportions, mean, median and range. We also used logistic regression analysis to check the possible association of characteristics of haematologists with survey results.
Results
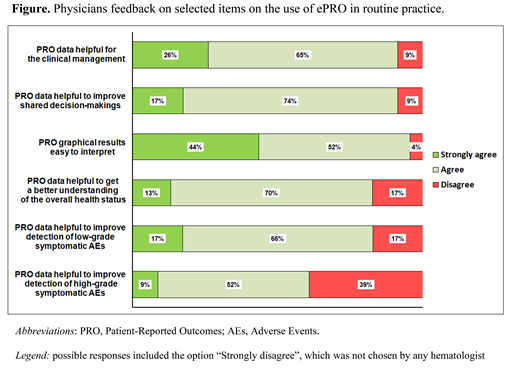
Of the 201 patients invited to participate between December 2020 and June 2021 (cut-off date for current analysis), 180 (90%) accepted to enter the ALLIANCE platform, currently activated in 19 centers. The median age of patients was 57 years (range 21-91) and 58% were males. The majority were diagnosed with chronic myeloid leukemia (n=32, 18%) and multiple myeloma (n=31, 17%) and were in stable disease (n=89, 49%). Twenty-three hematologists (44% males) with a median age of 42 years (range 31-63) and an average 17 years (range 5-34) of experience in clinical practice, completed the survey. The majority of physicians (78%) accessed the platform at least once per month (of whom 39% at least once per week), regardless the alerts sent by the system about patients' clinically relevant problems. The frequency of access on a regular basis was also independent of physician sex (p=0.393) and years of experience in clinical practice (p=0.404). Overall, 57% of hematologists discussed often or very often ePROs with their patients, while 83% and 61% deemed this information helpful to better identify symptomatic adverse events (AEs) of grade 1-2 or of grade 3-4, respectively (see figure). Also, 87% and 91% of hematologists found ePROs useful to improve physician-patient communication and the accuracy of documentation of symptomatic AEs (regardless of severity), respectively. Physicians' responses to selected items of the survey are reported in the figure.
Conclusions:
Current findings support the clinical utility, from the perspective of the treating physician, of integrating ePROs into routine cancer care of patients with hematologic malignancies.
Efficace: Takeda: Consultancy; Janssen: Consultancy; Abbvie: Consultancy, Other: Grants (to Institution); Amgen: Consultancy, Other: Grants (to Institution). Breccia: Bristol Myers Squibb/Celgene: Honoraria; Pfizer: Honoraria; Abbvie: Honoraria; Incyte: Honoraria; Novartis: Honoraria. Fazio: Janseen: Honoraria. Petrucci: Karyopharm: Honoraria, Other: Advisory Board; GSK: Honoraria, Other: Advisory Board; Amgen: Honoraria, Other: Advisory Board; Takeda: Honoraria, Other: Advisory Board; BMS: Honoraria, Other: Advisory Board; Janssen-Cilag: Honoraria, Other: Advisory Board; Celgene: Honoraria, Other: Advisory Board. Rigacci: Merck: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accomodations, Expenses; Gilead Science: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Menarini: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Tafuri: Roche: Research Funding; Celgene: Research Funding; Novartis: Research Funding. Siragusa: Novartis, CSL, Behring, Amgen, Novonoridsk, SOBI, Bayer: Consultancy, Honoraria, Speakers Bureau. Patriarca: Incyte: Honoraria; Takeda: Honoraria; Novartis: Honoraria; Amgen: Honoraria; Pfizer: Honoraria; Argenix: Honoraria. Luppi: Abbvie: Honoraria; Novartis: Honoraria; Sanofi: Honoraria; MSD: Honoraria; Gilead Science: Honoraria, Other: Travel grant; Daiichi-Sankyo: Honoraria; Jazz Pharma: Honoraria. Vignetti: Novartis: Honoraria; Incyte: Honoraria; Amgen: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal